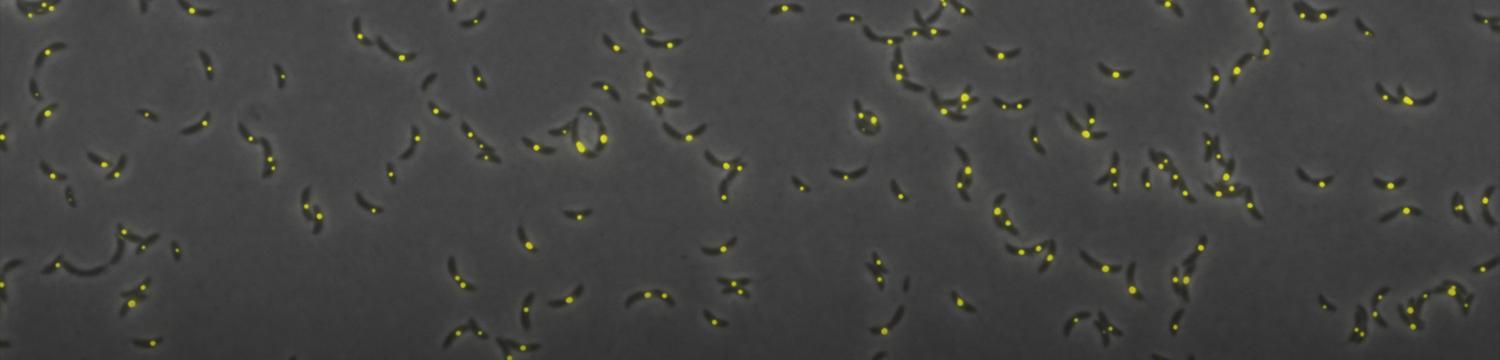
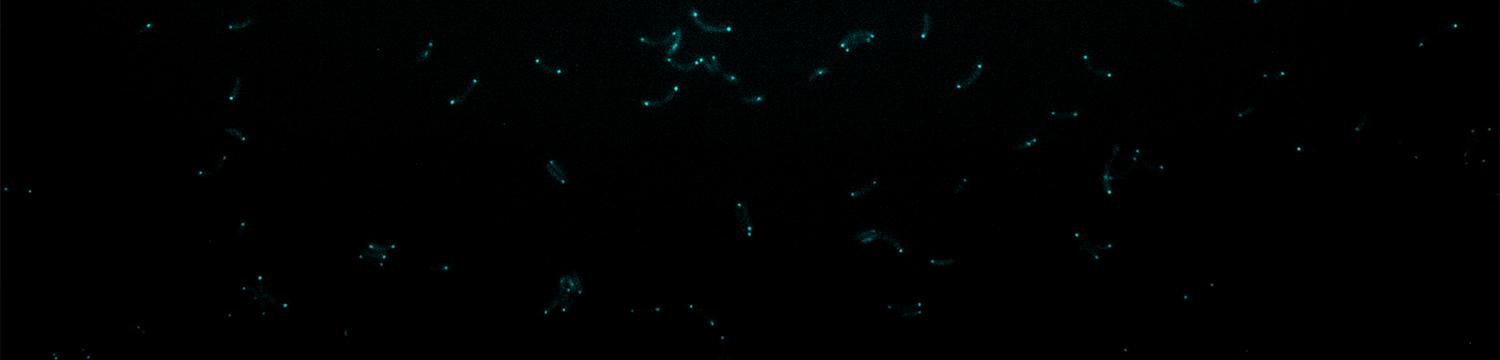
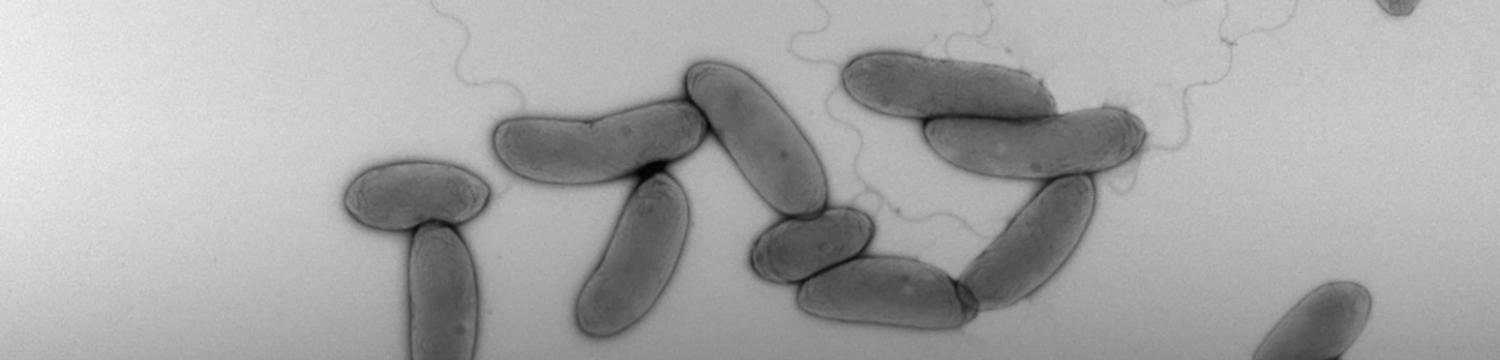
To survive to fluctuating environments, bacteria have evolved complex regulatory pathways that sense and respond adequately to changing conditions. One of these systems used by literally all bacteria is based on the alarmone (p)ppGpp. This second messenger accumulates upon starvation to essential elements (carbon, nitrogen, phosphorus, …), and the burst of (p)ppGpp modulates several cellular processes (transcription, metabolism, growth, cell cycle, virulence, …).
By combining genetic and biochemical approaches in our bacterial models, we (i) characterize mechanisms that trigger (p)ppGpp accumulation in response to nutritional stresses and (ii) look for direct (p)ppGpp targets.
We also characterize two-component signal transductions systems (TCS) involved in stress response.