Brucellosis, also known as “undulant fever”, “Mediterranean fever” or “Malta fever” is one of the most common bacterial zoonosis world-wide. Brucellosis affects a large range of mammals and is caused by facultative intracellular Gram-negative alphaproteobacteria of the genus Brucella. Brucella has evolved multiple strategies to evade immune response mechanisms to establish persistent infection and replication within host. Human brucellosis is transmitted through ingestion, inhalation, or contact of contaminated animal products with the conjunctiva or skin lesions. The majority of cases are caused by ingesting unpasteurized milk or cheese from infected goats or sheep. Person-to-person transmission is rare. The disease can be very insidious and may present in many atypical forms. In many patients the symptoms are mild and, therefore, the diagnosis may not be even considered. Indeed it should be noted that even in severe infections differential diagnosis can still be difficult. Brucella can cause a devastating multi-organ disease in humans with serious health complications in the absence of prolonged antibiotic treatment. Despite significant progress, the incidence of human brucellosis remains very high in endemic areas and is considered to be largely underestimated. In addition, Brucella species have been “weaponized” by several governments and are presently classified as category B threat agents. As complete eradication of Brucella would be unpractical due to its presence in a large range of wild mammals and because antibiotic treatment is costly and patients frequently suffer from resurgence of the bacteria, vaccination remains the most rational strategy to confer durable protection on populations living in endemic countries and professionals frequently exposed to Brucella. Unfortunately, there is currently no available human brucellosis vaccine as all commercially available animal vaccines are live vaccines which would cause disease in humans. Our group is working on several different aspects at the interface between the host and Brucella spp in experimental mouse models:
-
The identification of immune effector mechanisms implicated in the control of Brucella growth in vivo
Brucella spp. are characterized as stealthy pathogen that can persist lifelong in their hosts. Our objectives are (i) the identification of immune effector mechanisms implicated in the control of Brucella growth in vivo (ii) the characterization of reservoir cells allowing the persistence of Brucella in vivo. Infection is induced by intranasal inoculation in the mouse experimental model.
- MyD88-Dependent Activation of B220−CD11b+LY-6C+ Dendritic Cells during Brucella melitensis Infection
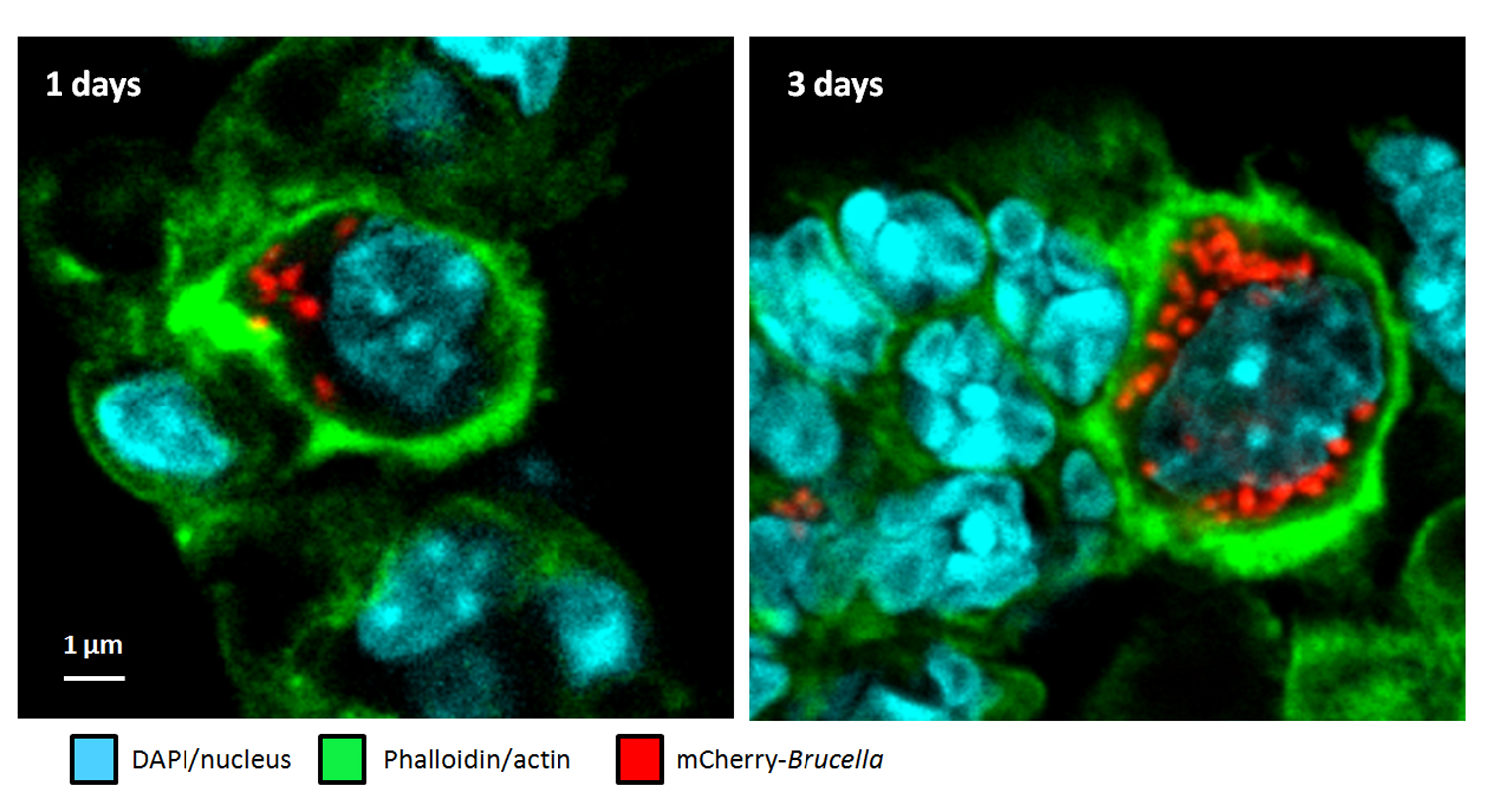
- In situ microscopy analysis reveals local innate immune response developed around Brucella infected cells in resistant and susceptible mice
https://journals.plos.org/plospathogens/article?id=10.1371/journal.ppat.1002575
- Crucial role of gamma interferon-producing CD4+ Th1 cells but dispensable function of CD8+ T cell, B cell, Th2, and Th17 responses in the control of Brucella melitensis infection in mice
https://journals.asm.org/doi/10.1128/IAI.00761-12
- Humoral immunity and CD4+ Th1 cells are both necessary for a fully protective immune response upon secondary infection with Brucella melitensis
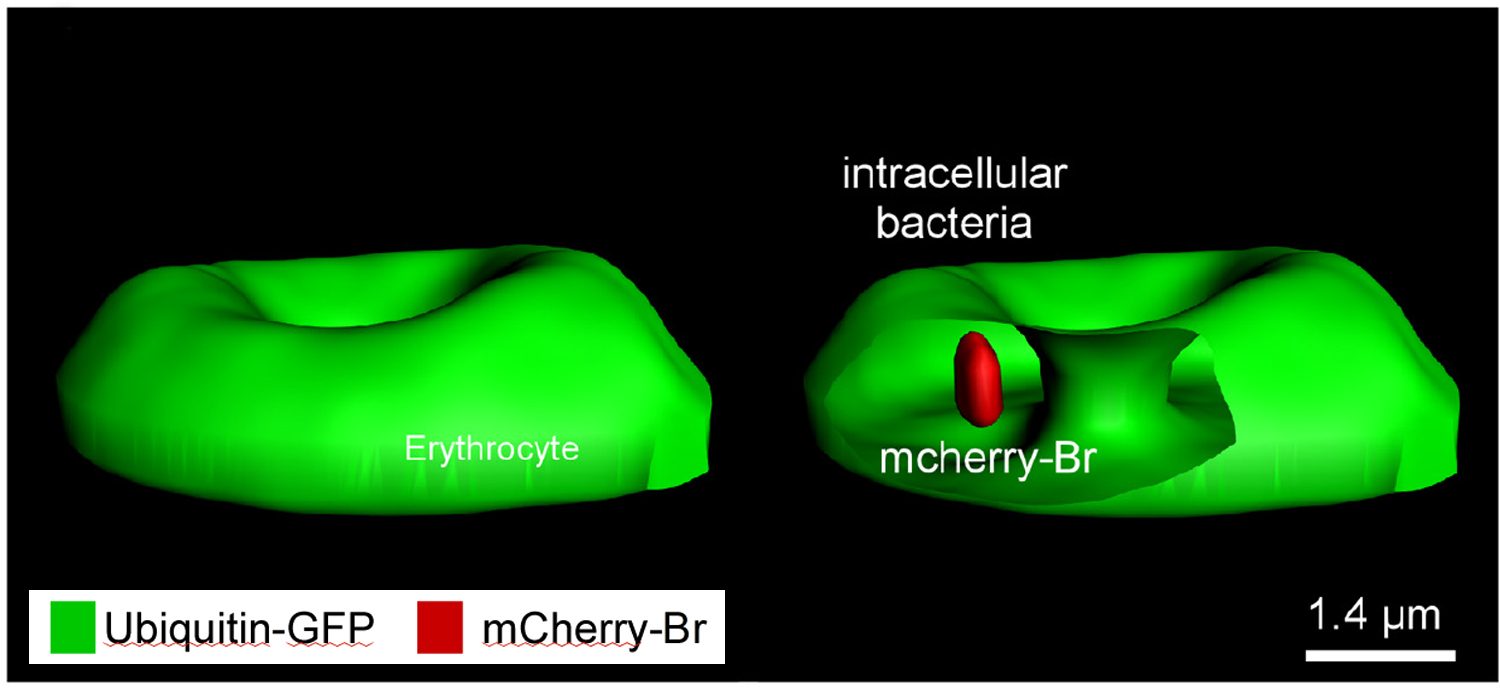
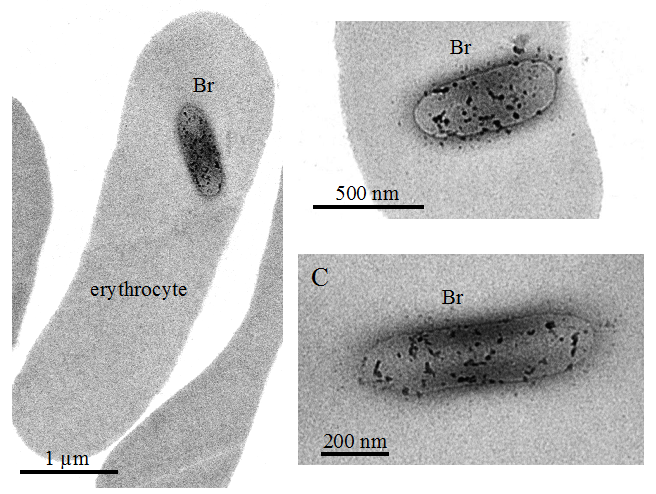
- Brucella melitensis invades murine erythrocytes during infection
https://journals.asm.org/doi/10.1128/IAI.01779-14
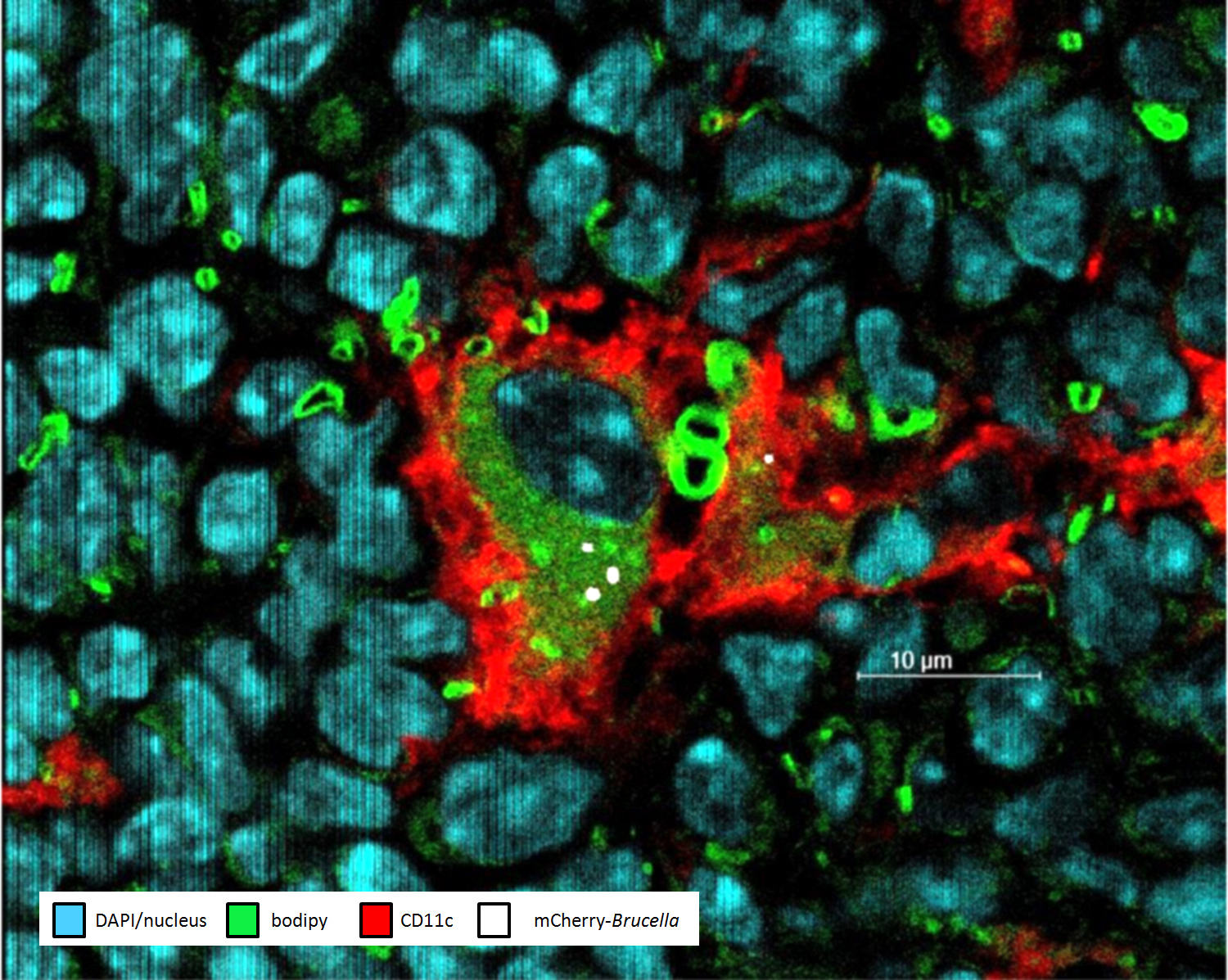
- In Situ Characterization of Splenic Brucella melitensis Reservoir Cells during the Chronic Phase of Infection in Susceptible Mice
https://journals.plos.org/plosone/article?id=10.1371/journal.pone.0137835
- Identification of Immune Effectors Essential to the Control of Primary and Secondary Intranasal Infection with Brucella melitensis in Mice
- Chronic Brucella Infection Induces Selective and Persistent Interferon Gamma-Dependent Alterations of Marginal Zone Macrophages in the Spleen
https://journals.asm.org/doi/10.1128/IAI.00115-17
- Route of Infection Strongly Impacts the Host-Pathogen Relationship
https://www.frontiersin.org/articles/10.3389/fimmu.2019.01589/full
- Aconitate decarboxylase 1 participates in the control of pulmonary Brucella infection in mice
https://pubmed.ncbi.nlm.nih.gov/34525130/
-
The impact of host immune status on the control of Brucella melitensis
In order to analyze the impact of unrelated infection or inflammatory disease on the control of Brucella melitensis infection, we have developed several model of cross-pathology. We studied the impact of Trypanosoma infection and allergic asthma (Derp1, Alternaria) on Brucella infection (cross-pathology models).
- Allergic Asthma Favors Brucella Growth in the Lungs of Infected Mice
https://www.frontiersin.org/articles/10.3389/fimmu.2018.01856/full
- Trypanosoma Infection Favors Brucella Elimination via IL-12/IFNγ-Dependent Pathways
https://www.frontiersin.org/articles/10.3389/fimmu.2017.00903/full
-
The identification of bacterial genes required to infect and persist in mice.
We used Transposon Sequencing (Tn-Seq) strategy to identify the bacterial genes indispensable to Brucella infection and persistence in mice.
- Genome-wide analysis of Brucella melitensis genes required throughout intranasal infection in mice
https://journals.plos.org/plospathogens/article?id=10.1371/journal.ppat.1010621