Publications
Lipopolysaccharide biosynthesis and traffic in the envelope of the pathogen Brucella abortus
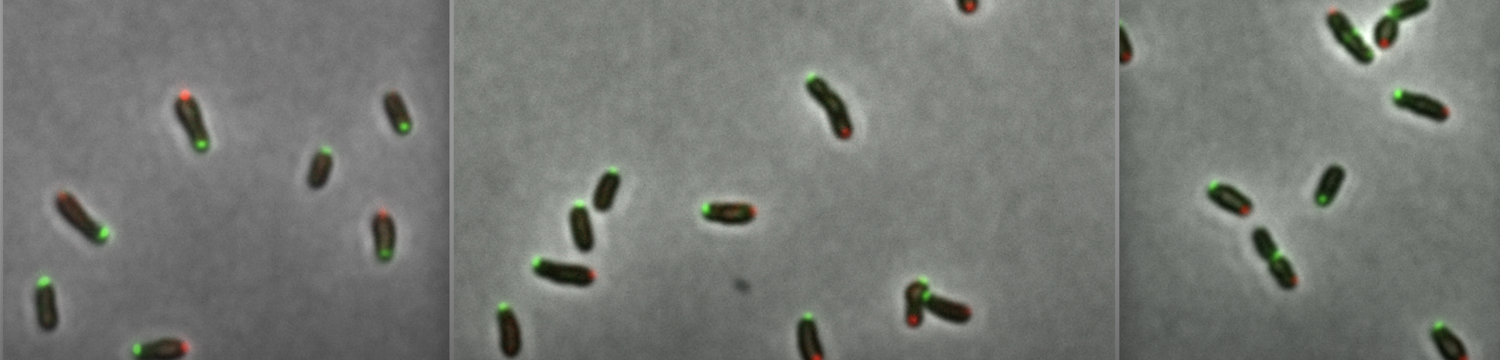
Lipopolysaccharide is essential for most Gram-negative bacteria as it is a main component of the outer membrane. In the pathogen Brucella abortus, smooth lipopolysaccharide containing the O-antigen is required for virulence. Being part of the Rhizobiales, Brucella spp. display unipolar growth and lipopolysaccharide was shown to be incorporated at the active growth sites, i.e. the new pole and the division site. By localizing proteins involved in the lipopolysaccharide transport across the cell envelope, from the inner to the outer membrane, we show that the lipopolysaccharide incorporation sites are determined by the inner membrane complex of the lipopolysaccharide transport system. Moreover, we identify the main O-antigen ligase of Brucella spp. involved in smooth lipopolysaccharide synthesis. Altogether, our data highlight a layer of spatiotemporal organization of the lipopolysaccharide biosynthesis pathway and identify an original class of bifunctional O-antigen ligases.
Post date: 2 years 11 months ago
A histidine auxotroph mutant is defective for cell separation and allows the identification of crucial factors for cell division in Brucella abortus
The pathogenic bacterium Brucella abortus invades and multiplies inside host cells. To grow inside host cells, B. abortus requires a functional histidine biosynthesis pathway. Here, we show that a B. abortus histidine auxotroph mutant also displays an unexpected chaining phenotype. The intensity of this phenotype varies according to the culture medium and is exacerbated inside host cells. Chains of bacteria consist of contiguous peptidoglycan, and likely result from the defective cleavage of peptidoglycan at septa. Genetic suppression of the chaining phenotype unearthed two essential genes with a role in B. abortus cell division: dipM and cdlP. Loss of function of dipM and cdlP generates swelling at the division site. While DipM is strictly localized at the division site, CdlP is localized at the growth pole and the division site. Altogether, the unexpected chaining phenotype of a hisB mutant allowed the discovery of new crucial actors in cell division in B. abortus.
Post date: 2 years 11 months ago
PdeA is required for the rod shape morphology of Brucella abortus
Cyclic-di-GMP plays crucial role in the cell cycle regulation of the α-Proteobacterium Caulobacter crescentus. Here we investigated its role in the α-Proteobacterium Brucella abortus, a zoonotic intracellular pathogen. Surprisingly, deletion of all predicted cyclic-di-GMP synthesizing or degrading enzymes did not drastically impair the growth of B. abortus, nor its ability to grow inside cell lines. As other Rhizobiales, B. abortus displays unipolar growth from the new cell pole generated by cell division. We found that the phosphodiesterase PdeA, the ortholog of the essential polar growth factor RgsP of the Rhizobiale Sinorhizobium meliloti, is required for rod shape integrity but is not essential for B. abortus growth. Indeed, the radius of the pole is increased by 31 ± 1.7% in a ΔpdeA mutant, generating a coccoid morphology. A mutation in the cyclic-di-GMP phosphodiesterase catalytic site of PdeA does not generate the coccoid morphology and the ΔpdeA mutant kept the ability to recruit markers of new and old poles. However, the presence of PdeA is required in an intra-nasal mouse model of infection. In conclusion, we propose that PdeA contributes to bacterial morphology and virulence in B. abortus, but it is not crucial for polarity and asymmetric growth.
Post date: 2 years 11 months ago
Beta-barrels covalently link peptidoglycan and the outer membrane in the alpha-proteobacterium Brucella abortus
Gram negative bacteria are surrounded by a cell envelope that comprises an outer membrane (OM) and an inner membrane (IM) which together delimit the periplasmic space, which contains peptidoglycan (PG). Covalent anchoring of the OM to the peptidoglycan is crucial for envelope integrity in Escherichia coli. When OM is not attached to PG the OM forms blebs and detaches from the cell. The Braun lipoprotein (Lpp) covalently attaches OM to PG but is only present in a small number of gamma-proteobacteria, which leaves the mechanism of OM-PG attachment in other species undetermined. We report that the OM is attached to the peptidoglycan by covalent crosslinks between the N-termini of integral OM beta-barrel-shaped proteins (OMPs) and the peptide stems of PG in the alpha-proteobacteria Brucella abortus and Agrobacterium tumefaciens. Crosslinking is catalyzed by L,D-transpeptidases and attached OMPs have a conserved alanyl-aspartyl motif at their N-terminus. Mutation of the aspartate in this motif prevents OMP crosslinking and results in OM membrane instability. The alanyl-aspartyl motif is conserved in OMPs from Rhizobiales, which means that it is feasible that OMP-PG crosslinks are widespread in alpha-proteobacteria
Post date: 5 years 2 weeks ago
Intracellular growth and cell cycle progression are dependent on (p)ppGpp synthetase/hydrolase in Brucella abortus
Brucella abortus is a pathogenic bacterium able to proliferate inside host cells. During the first steps of its trafficking, it is able to block the progression of its cell cycle, remaining at the G1 stage for several hours, before it reaches its replication niche. We hypothesized that starvation mediated by guanosine tetra- or penta-phosphate, (p)ppGpp, could be involved in the cell cycle arrest. Rsh is the (p)ppGpp synthetase/hydrolase. A B. abortus ∆rsh mutant is unable to grow in minimal medium, it is unable to survive in stationary phase in rich medium and it is unable to proliferate inside RAW 264.7 macrophages. A strain producing the heterologous constitutive (p)ppGpp hydrolase Mesh1b is also unable to proliferate inside these macrophages. Altogether, these data suggest that (p)ppGpp is necessary to allow B. abortus to adapt to its intracellular growth conditions. The deletion of dksA, proposed to mediate a part of the effect of (p)ppGpp on transcription, does not affect B. abortus growth in culture or inside macrophages. Expression of a gene coding for a constitutively active (p)ppGpp synthetase slows down growth in rich medium and inside macrophages. Using an mCherry–ParB fusion able to bind to the replication origin of the main chromosome of B. abortus, we observed that expression of the constitutive (p)ppGpp synthetase gene generates an accumulation of bacteria at the G1 phase. We thus propose that (p)ppGpp accumulation could be one of the factors contributing to the G1 arrest observed for B. abortus in RAW 264.7 macrophages.
Post date: 5 years 2 weeks ago
Occurrence and repair of alkylating stress in the intracellular pathogen Brucella abortus
Over the last decades, many in vitro studies led to a better understanding of the causes and consequences of alkylating stress on DNA, as well as to the repair mechanisms selected by prokaryotic cells to face it. It has been proposed that pathogenic bacteria have to cope with alkylating stress inside host cells, but this assumption has never been demonstrated. Here, using single cell reporter systems, we show that the class III pathogen Brucella abortus does encounter alkylating stress during the first hours of macrophages infection. Direct repair and base excision repair pathways were identified to be needed by B. abortus to face this stress in vitro and in a mice infection model. In addition, B. abortus relies on distinct strategies than Escherichia coli to control DNA repair gene expression, since we found that some of these genes are under the control of the conserved cell-cycle transcription factor GcrA.
Post date: 5 years 2 weeks ago
Localized incorporation of outer membrane components in the pathogen Brucella abortus
The zoonotic pathogen Brucella abortus is part of the Rhizobiales, which are alpha-proteobacteria displaying unipolar growth. Here, we show that this bacterium exhibits heterogeneity in its outer membrane composition, with clusters of rough lipopolysaccharide co-localizing with the essential outer membrane porin Omp2b, which is proposed to allow facilitated diffusion of solutes through the porin. We also show that the major outer membrane protein Omp25 and peptidoglycan are incorporated at the new pole and the division site, the expected growth sites. Interestingly, lipopolysaccharide is also inserted at the same growth sites. The absence of long-range diffusion of main components of the outer membrane could explain the apparent immobility of the Omp2b clusters, as well as unipolar and mid-cell localizations of newly incorporated outer membrane proteins and lipopolysaccharide. Unipolar growth and limited mobility of surface structures also suggest that new surface variants could arise in a few generations without the need of diluting pre-existing surface antigens.
Post date: 7 years 1 month ago
CtrA controls cell division and outer membrane composition of the pathogen Brucella abortus
Post date: 8 years 11 months ago
Brucella abortus Cell Cycle and Infection Are Coordinated
Trends Microbiol. (12):812-21.
Post date: 9 years 9 months ago
G1-arrested newborn cells are the predominant infectious form of the pathogen Brucella abortus
Several intracellular pathogens, such as Brucella abortus, display a biphasic infection process starting with a non-proliferative stage of unclear nature. Here, we study the cell cycle of B. abortus at the single-cell level, in culture and during infection of HeLa cells and macrophages. The localization of segregation and replication loci of the two bacterial chromosomes indicates that, immediately after being engulfed by host-cell endocytic vacuoles, most bacterial cells are newborn. These bacterial cells do not initiate DNA replication for the next 4 to 6h, indicating a G1 arrest. Moreover, growth is completely stopped during that time, reflecting a global cell cycle block. Growth and DNA replication resume later, although bacteria still reside within endosomal-like compartments. We hypothesize that the predominance of G1-arrested bacteria in the infectious population, and the bacterial cell cycle arrest following internalization, may constitute a widespread strategy among intracellular pathogens to colonize new proliferation niches.
Post date: 10 years 4 months ago
BtaE, an adhesin that belongs to the trimeric autotransporter family, is required for full virulence and defines a specific adhesive pole of Brucella
Immun. 81, 996-1007
Post date: 10 years 4 months ago
The Brucella pathogens are polarized bacteria
Microbes and Infection 15, 998-1004
Post date: 10 years 4 months ago
The histidine kinase PdhS controls cell cycle progression of the pathogenic alphaproteobacterium Brucella abortus
J. Bacteriol. 194, 5305-5314.
Post date: 10 years 4 months ago
Characterization of a Brucella abortus mutant defective for the association with endoplasmic reticulum exit sites in HeLa cells
Microbiology 158, 2610-2618
Post date: 10 years 4 months ago
Structural analysis of Brucella abortus RicA substitutions that do not impair interaction with human Rab2 GTPase
BMC Biochemistry 13, 16
Post date: 10 years 4 months ago
Identification of a Brucella spp. secreted protein specifically interacting with human Rab2 small GTPase
Cell. Microbiol. 13, 1044-1058
Post date: 10 years 4 months ago
The alkylation response protein AidB is localized at the new poles and constriction sites in Brucella abortus
BMC Microbiol. 11, 257.
Post date: 10 years 4 months ago
RpoE1, an extracytoplasmic function sigma factor, is a repressor of the flagellar system in Brucella melitensis
Microbiology 157, 1263-1268.
Post date: 10 years 4 months ago
PdhS, an old pole-localized histidine kinase, recruits the fumarase FumC in Brucella abortus
J. Bacteriol. 192, 3235-3239.
Post date: 10 years 4 months ago
Identification of the essential Brucella melitensis porin Omp2b as a suppressor of Bax-induced cell death in yeast in a genome-wide screening
PLoS ONE 5, e13274.
Post date: 10 years 4 months ago
Aggregates of soluble and folded proteins as intermediate state of inclusion body formation in Escherichia coli
BMC Microbiology 10, 248.
Post date: 10 years 4 months ago
Global analysis of quorum sensing targets in the intracellular pathogen Brucella melitensis 16M
J. Proteome Res. 9, 3200-3217.
Post date: 10 years 4 months ago
Functional characterization of the incomplete phosphotransferase system (PTS) of the intracellular pathogen Brucella melitensis
PLoS ONE 5, e12679.
Post date: 10 years 4 months ago
Intracellular rescuing of a B. melitensis 16M virB mutant by co-infection with a wild type strain
Microb. Pathog. 45, 134-141.
Post date: 10 years 4 months ago
The asymmetric distribution of the essential histidine kinase PdhS indicates a differentiation event in Brucella abortus
EMBO J. 26, 1444-1455.
Post date: 10 years 4 months ago
Mutations of the Quorum sensing-dependent regulator VjbR lead to drastic surface modifications in Brucella melitensis
J. Bacteriol. 189, 6035-6047.
Post date: 10 years 4 months ago
NnrA is required for full virulence and regulates several Brucella melitensis denitrification genes
J. Bacteriol. 188, 1615-1619.
Post date: 10 years 4 months ago
A RpoH-like heat shock sigma factor is involved in stress response and virulence in Brucella melitensis 16M
J. Bacteriol. 188, 7707-7710
Post date: 10 years 4 months ago
The stringent response mediator Rsh is required for Brucella spp. virulence, and regulates expression of the type IV secretion system VirB
Cell. Microbiol. 8, 1791-1802.
Post date: 10 years 4 months ago
FtcR is a new master regulator of the flagellar system of Brucella melitensis 16M with homologs in Rhizobiaceae
J. Bacteriol. 189, 131-141.
Post date: 10 years 4 months ago
Systematic mutagenesis of Brucella melitensis 16M reveals a major role for GntR regulators in the control of virulence
Infect. Immun. 73, 5578-5586.
Post date: 10 years 4 months ago
A quorum sensing regulator controls expression of both the type four secretion system and the flagellar apparatus of Brucella melitensis
Cell. Microbiol. 7, 1151-1161.
Post date: 10 years 4 months ago
Generation of the Brucella melitensis ORFeome version 1.1
Genome Res. 14, 2201-2206.
Post date: 10 years 4 months ago
Morphological and functional asymmetry in alpha-proteobacteria
Trends Microbiol. 12, 361-365.
Post date: 10 years 4 months ago
The Ton system, an ABC transporter, and a universally conserved GTPase are involved in iron utilization by Brucella melitensis 16M
Infect. Immun. 72, 5783-5790.
Post date: 10 years 4 months ago
Attenuated signature-tagged mutagenesis mutants of Brucella melitensis identified during the acute phase of infection in mice
Infect. Immun. 71, 7053-7060.
Post date: 10 years 4 months ago
Plasticity of transcriptional regulation network among alpha-proteobacteria is supported by the identification of CtrA targets in Brucella abortus
Mol. Microbiol. 43, 945-960. IF: 6.3
Post date: 10 years 4 months ago
Prediction of proteins 3D structures
Bioinformatics 18, 1250-1256. IF: 3.4
Post date: 10 years 4 months ago
Polar growth in the Alphaproteobacterial order Rhizobiales
Proc Natl. Acad. Sci. USA 109, 1697-1701
Post date: 10 years 4 months ago