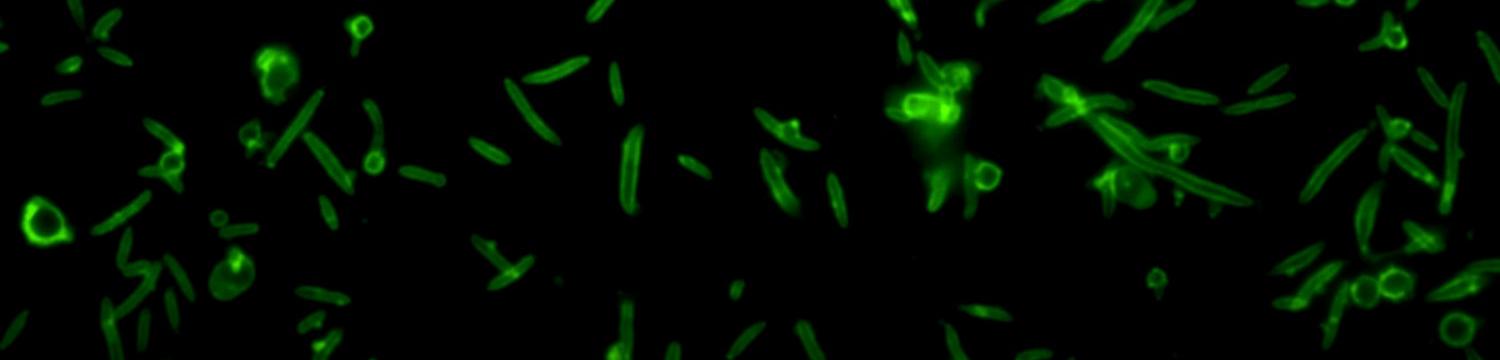
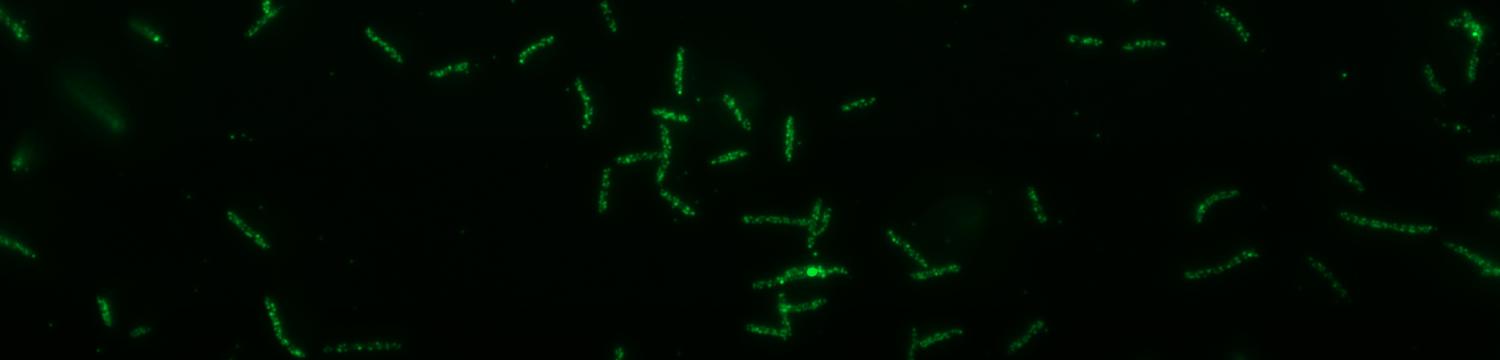
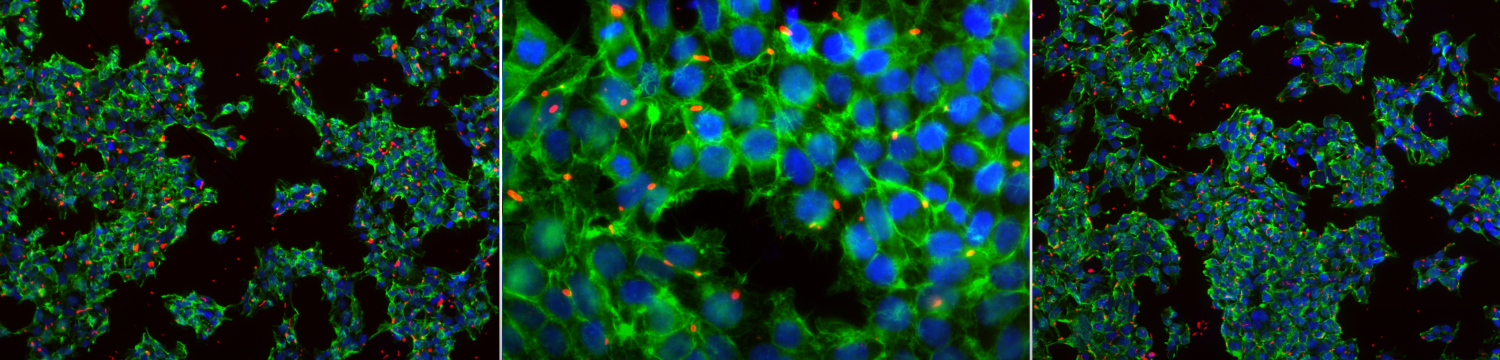
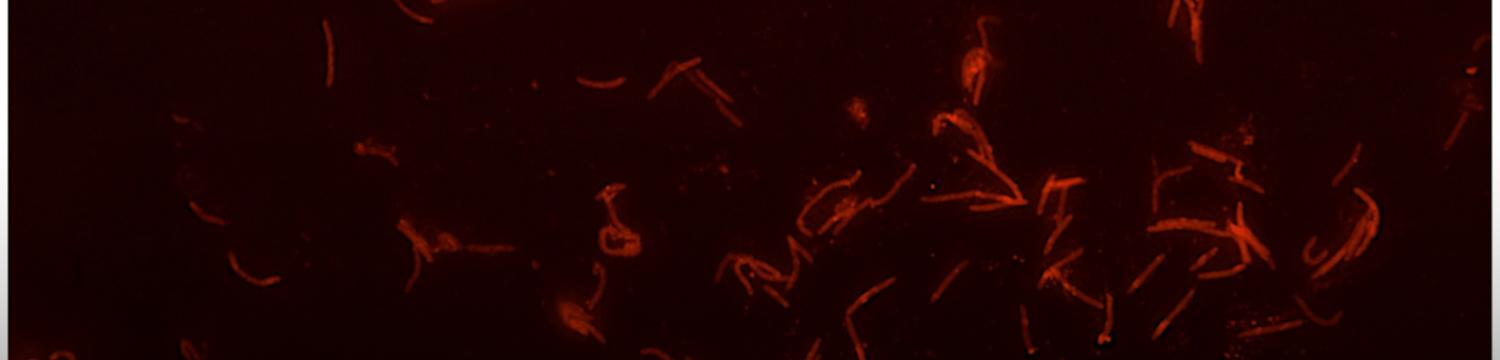
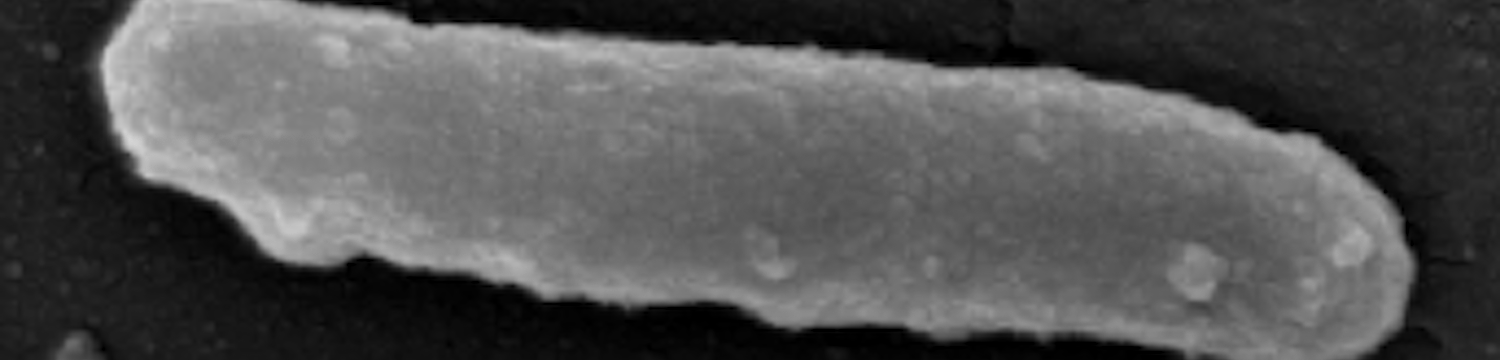
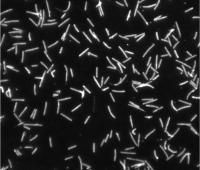
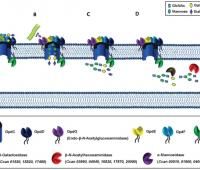
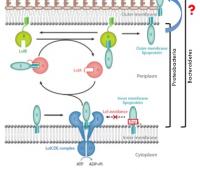
The surface structures of bacteria are essential for their survival in the host and hence their pathogenicity. They may represent a protection against the attack of complement and against phagocytosis but at the same time, they are an Achille's heel since the innate immunity system has evolved to recognize them to trigger inflammation. We investigate the structure and role of the different surface structures of Capnocytophaga canimorsus, in collaboration with U. Zähringer (Borstel, D), I. Sadovskaya (Boulogne-sur-mer, F) and E. Maes (Lille, F).
Publications:
- Renzi, F*, Hess, E*, Dol, M, Koudad, D, Carlier, E, Ohlén, M, Moore, E & Cornelis, GR 2018, Capsular serovars of virulent Capnocytophaga canimorsus are shared by the closely related species C. canis and C. cynodegmi Emerging Microbes & Infections, vol. 7, no. 1, 124, pp. 124. DOI: 10.1038/s41426-018-0126-x * equal contribution.
- Hess, E*, Renzi, F*, Karhunen, P*, Dol, M, Lefevre, A, Antikainen, J, Carlier, E, Hästbacka, J & Cornelis, G 2018, Capnocytophaga canimorsus Capsular Serovar and Disease Severity, Helsinki Hospital District, Finland, 2000–2017 Emerging Infectious Diseases, vol. 24, no. 12, pp. 2195-2201. DOI: 10.3201/eid2412.172060 * equal contribution.
- Hess, E*, Renzi, F*, Koudad, D, Dol, M & Cornelis, G 2017, Identification of virulent Capnocytophaga canimorsus isolates by capsular typing Journal of clinical microbiology, vol. 55, no. 6, pp. 1902-1914. DOI: 10.1128/JCM.00249-17 * equal contribution
- Renzi, F, Ittig, SJ, Sadovskaya, I, Hess, E, Lauber, F, Dol, M, Shin, H, Mally, M, Fiechter, C, Sauder, U, Chami, M & Cornelis, G 2016, Evidence for a LOS and a capsular polysaccharide in Capnocytophaga canimorsus Scientific Reports. https://doi.org/10.1038/srep38914
- Renzi F., Zähringer U., Chandler C.E., Ernst R.K., Cornelis G.R., Ittig S.J. Modification of the 1-phosphate group during biosynthesis of Capnocytophaga canimorsus lipid A. Infection and Immunity. 2015 Dec 7. pii: IAI.01006-15.
- Zähringer U., Ittig S., Lindner B., Moll H., Schombel U., Gisch N., Cornelis G.R. NMR-based structural analysis of the complete rough-type lipopolysaccharide isolated from Capnocytophaga canimorsus. J Biol Chem. 2014 Aug 22;289(34):23963-76. doi: 10.1074/jbc.M114.571489. Epub 2014 Jul 2.
- Ittig, S., Lindner, B., Stenta, M., Manfredi, P., Zdorovenko, E., Knirel, Y., Dal Peraro, M., Cornelis, G.R, Zähringer, U. The Lipopolysaccharide from Capnocytophaga canimorsus Reveals an Unexpected Role of the Core-Oligosaccharide in MD-2 Binding. PLoS pathogens 2012, 8(5), e100266
- Shin H., Mally M., Meyer S., Fiechter C.,Paroz C., Zähringer U., Cornelis G.R. Resistance of Capnocytophaga canimorsus to killing by human complement and polymorphonuclear leukocytes. Infection and Immunity 2009, 77(6): 2262–2271. Epub Mar 23, 2009