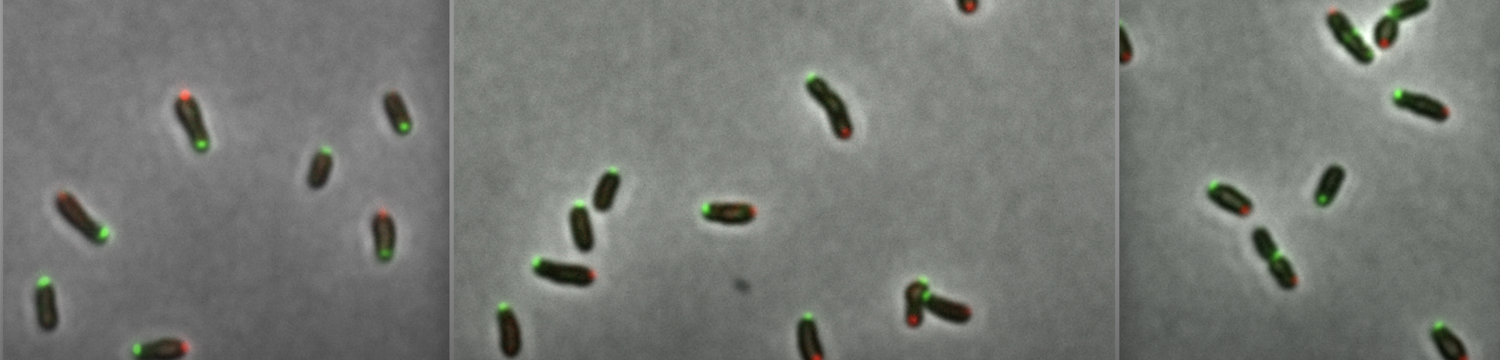
In our team, we study essential processes in the molecular biology of the pathogen Brucella abortus, a zoonotic pathogen with a strong intracellular tropism. During its infectious cycle, B. abortus is expected to meet different stresses to which the bacterium is adapted. Beyond its ability to survive these stresses, the bacterium needs to grow in its host before being transmitted to the next host. Therefore, growth and stress response are two key factors for the success of B. abortus infectious cycle. A better knowledge of the associated molecular mechanisms will improve our basic knowledge allowing the next generation to choose the best possible treatments in the future.
Our current research lines include :
• adaptation to envelope stress as well as high copper concentrations
• envelope growth, particularly the localization of lipopolysaccharide biosynthesis
• polarity of virulence factors
Five selected publications over the last 10 years (a complete list is available in the Publications section) :
• Servais, C., Vassen, V., Verhaeghe, A., Küster, N., Carlier, E., Phégnon, L., Mayard, A., Auberger, N., Vincent, S., De Bolle, X. (2022) Lipopolysaccharide biosynthesis and traffic in the envelope of the pathogen Brucella abortus. Nature Communications 14, 911.
• Godessart, P., Lannoy, A., Dieu, M., Van der Verren, S.E., Soumillion, P., Collet, J.-F., Remaut, H., Renard, P., De Bolle, X. (2021) Beta-barrels covalently link peptidoglycan and the outer membrane in the alpha-proteobacterium Brucella abortus. Nature Microbiology 6, 27-33.
• Vassen, V., Valotteau, C., Feuillie, C., Formosa-Dague, C., Dufrêne, Y.F., De Bolle, X. (2019) Localized incorporation of outer membrane components in the pathogen Brucella abortus. EMBO Journal 38, e100323.
• Poncin, K., Roba, A., Jimmidi, R., Potemberg, G., Fioravanti, A., Francis, N., Willemart, K., Zeippen, N., Machelart, A., Biondi, E.G., Muraille, E., Vincent, S.P. and De Bolle, X. (2019) Occurrence and repair of alkylating stress in the intracellular pathogen Brucella abortus. Nature Communications 10, 4847.
• Deghelt, M., Mullier, C., Sternon, J.F., Francis, N., Laloux, G., Dotreppe, D., Van der Henst, C., Jacobs-Wagner, C., Letesson, J.J., De Bolle, X. (2014) G1-arrested newborn cells are the predominant infectious form of the pathogen Brucella abortus. Nature Communications 5, 4366.